Age-Related Macular Degeneration: What is Dry AMD, and How is Lineage Cell Therapeutics Changing the Game?
Previously Published to Benzinga: The following post was written and/or published as a collaboration between Benzinga’s in-house sponsored content team and a financial partner of Benzinga.
Age-related macular degeneration (AMD) is a disease originating in the retina, which may worsen over time, according to WebMD. It’s said to be the leading cause of severe, permanent vision loss in people older than 60.
When the macula — the small central portion of the retina — wears down, the light-sensing nerve tissue at the back of the eye begins to deteriorate. While it doesn’t always cause complete blindness, severe vision problems such as loss of central vision may occur. As the condition worsens, these light-sensitive cells will thin out and eventually die off. They do not regenerate.
Early Treatment of AMD can Prevent Vision Loss Later in Life
There are 2 forms of this disease. People with macular degeneration will either experience the dry (atrophic) form of macular degeneration or the wet (neovascular) form. The American Academy of Ophthalmology (AAO) reports that about 80% (8 out of 10) of people with AMD have the dry form.
This disease currently has no cure or FDA-approved treatments. Most therapeutic efforts today are directed to delaying the degenerative process to give one’s eyes just a little bit more time. None of these approaches has been successful in clinical trials.
Now, thanks to Lineage Cell Therapeutics Inc. (AMEX: LCTX), there is a lot more hope for the future of folks vulnerable to this disease. Lineage’s approach is focused on a retinal pigment epithelium (RPE) cell transplant, which aims to replace damaged retinal cells in patients who suffer from retinal lesions or degeneration.
If dry AMD goes untreated, it can evolve into a form of wet AMD, which is far less common but way more serious. This condition is when new, abnormal blood vessels form under the retina, often leaking blood and other fluids while causing scarring of the central retina nerves. Those who show signs of wet AMD will lose their vision much faster than those with dry AMD.
Who is Lineage Cell Therapeutics?
Lineage Cell Therapeutics is exploring potential paths toward regulatory submission for macular cell therapy and transplantation. Based on the findings of a previous study, OpRegen received a fast-track designation from the FDA. After a series of new testing and assessment, the company is working toward taking its product to market.
Last month, Lineage released the results of a Phase 1/2a clinical study of its lead product candidate, OpRegen®, that demonstrated the functional benefits to each patient’s vision, a significant improvement in macular degeneration and most notably, the only known cases of retinal tissue restoration in dry AMD patients, as verified by independent experts, as well as through multiple tests and imaging technologies.
What Lineage is Doing for Patients with Dry AMD
The company has 3 clinical programs in the cellular therapy arena, which includes 3 different allogeneic, or “off-the-shelf” products. The OpRegen program serves to transplant and replace RPE cells that cause dry age-related macular degeneration. Other programs focus on treating and repairing spinal cord injuries, as well as treating various cancers.
In addition to measuring visual acuity, ongoing OpRegen clinical studies demonstrate positive structural changes in the eye as well as positive changes in retinal thickness, organization and overall health of the retina in areas of potential atrophy via cellular transplant therapy. The company uses multiple imaging techniques to affirm detailed information on a patient’s retina.
According to a recent study, OpRegen is currently being evaluated in an open-label, dose-escalation safety and efficacy study in patients with advanced dry AMD with GA. Patients received a single injection of retinal pigment epithelium cells grown from an established cell line.
New findings from Lineage support the view that atrophic AMD is not an irreversible, degenerative condition that one cannot recover from. Despite previous notions, some portion of diseased retinal tissue may actually be able to regenerate and heal over time, changing the game for those who have lost their eyesight to both versions of macular degeneration.
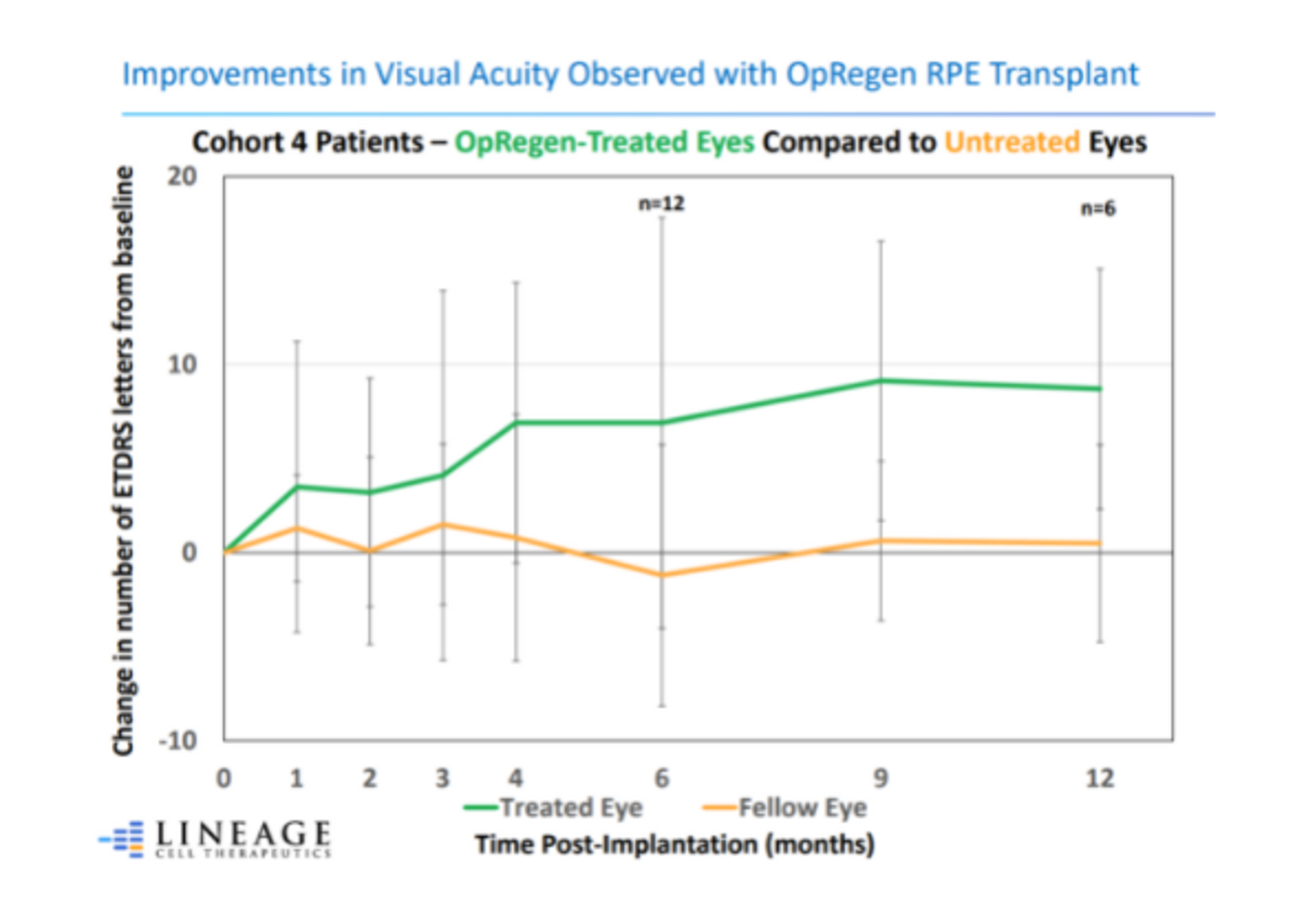
To date, the company has carried out these studies in 24 patients. The 12 patients treated in Cohort 4 had better baseline vision and smaller areas of GA at baseline than earlier cohorts. Positive results were achieved in the first-ever retinal restoration of vision loss in patients — with the earliest transplant taking place more than 3 years ago.
OpRegen is currently being evaluated in a Phase 1/2a open-label, dose-escalation safety and efficacy study in patients with advanced dry AMD with GA. These patients receive a single injection of retinal pigment epithelium cells grown from an established cell line.
The Study Placed 24 Patients into 4 Different Cohorts
The first 3 Cohort groups consisted of legally blind patients with a 20/200 vision or worse. The 4th Cohort groups 12 patients with vision between 20/65 and 20/250 and a smaller mean area of GA.
Cohort 4 also includes patients treated with a new thaw-and-inject form of OpRegen, which can be shipped directly to sites and used immediately upon thawing. This would ultimately remove the complications and logistics of having to use a dose preparation facility.
The study evaluated both the safety and tolerability of OpRegen treatments, as it coincided with the number of incidences, the frequency of treatment and any emergence of adverse events.
It further assessed the efficacy of the OpRegen therapies by determining the changes in ophthalmological parameters measured by various methods of primary clinical relevance. OpRegen has been well-tolerated to date, and no new, unexpected or adverse ocular events have been reported in the company’s most recent update.
The Results of the Study Determined
Three patients with a confirmed history of GA growth have demonstrated evidence of retinal tissue restoration. They continue to demonstrate key areas of retinal restoration, as of their last assessment, which ranges from between 6 months to approximately 3 years after treatment.
Notably, on OCT analyses, the 1st patient with evidence of retinal restoration and confirmed history of GA growth, has demonstrated zero growth in the area of atrophy almost 3 years following treatment with OpRegen. This is unprecedented because of the progressive nature of the disease.
Key takeaways include:
- Of all Cohort 4 better vision patients, 83% continued to exhibit stable or improved best corrected visual acuity (BCVA) for at least 6 months post-treatment, whereas visual acuity declined in the majority of untreated eyes.
- The 3 patients with evidence of retinal tissue restoration continue to demonstrate areas of restoration with sustained visual acuity and improvement as of their last clinical visit.
- A patient with confirmed atrophy growth at the baseline has had zero progression for almost 3 full years.
- The company intends to meet with the FDA in the Q4 2021 to discuss a plan for the further clinical development of OpRegen.
Why Invest in Lineage Cell Therapeutics?
The field of cell therapy is poised for explosive growth and scalable allogeneic, or “off-the-shelf” approaches have the ability to provide significant commercial advantages over autologous or patient-derived methods. Lineage’s objective is to be positioned for rapid growth of this emerging branch of medicine by providing evidence that “off-the-shelf” cells can generate safety and efficacy data in large commercial opportunities where small molecules have not succeeded.
The global market for macular degeneration is poised to double by 2028. Global sales for the 2 leading wet AMD therapies were more than $10 billion in 2019. The global cell therapy market was valued at $7.8 billion in 2020 and is expected to also expand at a compound annual growth rate (CAGR) of 14.5% into 2028. Over this period, the entire cell therapy market is projected to be worth more than $23 billion.
AMD is the leading cause of irreversible vision loss in the U.S. To learn more about Lineage’s OpRegen Treatment Program and the results of the clinical study, visit https://www.genetherapylive.com/view/opregen-sustained-efficacy-treating-dry-amd.
For more information about Lineage Cell Therapeutics and how to invest, visit https://www.lineagecell.com, or follow the company on Twitter @LineageCell or at @CEO_Culley.
Attend the Lineage Q2 Earnings Call
Lineage plans to report Q2 earnings and the results of ongoing operations following the close of U.S. markets on Thursday, August 12, 2021. The management team is also set to host an open webcast and conference call at 4:30 p.m. EST to discuss Q2 2021 financial results and provide a business update.
Interested parties may access the conference call by dialing:
- U.S.: (866) 888-8633
- Canada: (636) 812-6629
All callers should request the “Lineage Cell Therapeutics Call’ when prompted. A live webcast will be available within the investors section of Lineage’s corporate website.
The preceding post was written and/or published as a collaboration between Benzinga’s in-house sponsored content team and a financial partner of Benzinga. Although the piece is not and should not be construed as editorial content, the sponsored content team works to ensure that any and all information contained within is true and accurate to the best of their knowledge and research. This content is for informational purposes only and not intended to be investing advice.